

Technology
Core Technology
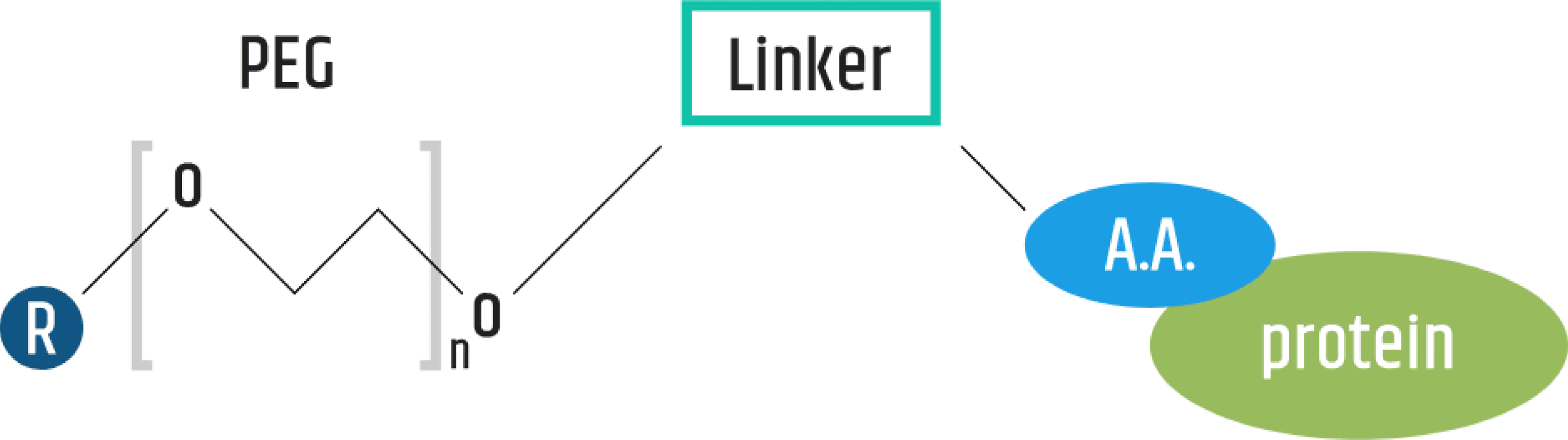
In the field of protein drug design, the process of pegylation of the therapeutic protein preserves its biological activity by targeting the PEG polymer (polyethylene glycol) at a specific and defined region on the protein. PharmaEssentia's pegylation technology platform is designed to increase the protein drug's efficacy by prolonging its circulation in the bloodstream.
Utilizing its innovative 40K PEG and its novel pegylation technology platform by combining protein engineering and PEG-related chemistry, PharmaEssentia creates novel products for better disease treatment. Compared with the similar drugs on the market, the long-acting protein drugs produced by the technology possess the most effective PK/PD data as well as reduced side effects, as these PEG-proteins are designed as predominantly single forms without other isomers which may induce severe side effects.
Ropeginterferon alfa 2b (P1101)
PharmaEssentia's novel pegylation technology platform has yielded our lead product, Ropeginterferon alfa-2b, which is a novel, long-acting, mono-pegylated proline interferon with improved pharmacokinetic properties allowing once every two weeks administration offering improved tolerability and convenience.

A new approach
Interferons are among the most recognized and well-studied therapeutic agents for a range of serious diseases. These proteins help the immune system fight certain infections, diseases, and cancers. However, historically, use of interferons in treating MPNs and other diseases has been limited due to difficulties with administration and a challenging tolerability. In addition to IFN, we are developing new cytokine therapies leveraging our proprietary pegylation technology with the potential to be best-in-class.
PharmaEssentia has worked to address these shortcomings with a proprietary PEGylation technology, with the goal of giving physicians new and improved therapeutic options.

A novel technology platform
Leveraging pioneering expertise in interferons, our Research and Development team developed an innovative PEGylation technology designed to improve tolerability, dosing convenience, and efficacy in MPNs. This led to the successful development of ropeginterferon alfa-2b (P1101), a mono-pegylated proline interferon. This has now been approved for BESREMi in PV and is being investigated in other MPNs, including ET.
Our proprietary, site-specific PEGylation technology has the potential to provide long-lasting biologics applicable for a wide array of indications.
We discover new treatments and deliver improvements to as many people as possible.
At PharmaEssentia, we not only commit to patients exploring new ways to extend people's lives,
but also seeking collaboration and provide supports to healthcare professionals.

Global research capabilities
Our research and development teams have extensive capabilities in analytical science, chemistry, cell culture, protein engineering, pharmaceutical science, and process development. We complement our R&D facilities in Taiwan with the PharmaEssentia Innovation Research Center (PIRC) in the U.S. Together, they form a global innovation bridge that accelerates research and development across borders.
PharmaEssentia has built strong partnerships with world-class collaborators, ranging from university research institutes to biotechnology and pharmaceutical companies. Our scientific leaders are actively engaged in shaping the scientific discourse in the hematology/oncology field.
An expanding pipeline focused on MPNs and other hematologic malignancies
PharmaEssentia's clinical pipeline includes candidates with a focus across a range of MPNs and other hematologic malignancies .
We intend to expand into other areas where our innovative technology can address needs and support improved outcomes.
Accelerating development programs
Our R&D identifies and advances scientific breakthroughs that make new therapies for rare cancers possible, helping to improve patients’ health and quality of life. We’re currently focused on three main areas of drug discovery:
Ropeginterferon alfa-2b has Orphan Drug designation in the United States of America and the European Union. We plan to commercializeRopeginterferon alfa-2b in North and South America, as well as Asia. We have exclusively licensed the rights to Ropeginterferon alfa-2b to AOP Orphan for European, Commonwealth of Independent States (CIS), and Middle Eastern markets for the development and commercialization in the field of Myeloproliferative Neoplasms (MPNs).

BESREMi® for polycythemia vera (PV)
BESREMi is the only FDA-approved treatment indicated for adults with polycythemia vera (PV) that targets the source of the disease.

Erik Sampson
Director of Translational Pharmacology
"At PIRC, I help translate scientific discoveries into potential therapies, partnering with our global R&D teams in Boston and Taiwan. Together, we’re advancing development programs in oncology, hematology, and immunology that aim to bring new hope to patients with rare cancers."