

Manufacturing
State-of-the-art manufacturing
Built in 2012, our 43,000-square-foot manufacturing center in Taiwan is a cGMP facility fully equipped with leading-edge biopharmaceutical production technology. It has been inspected and certified by the European Medicines Agency and the Taiwan Food and Drug Administration, and is compliant with all U.S. Food and Drug Administration requirements. The facility combines polyethylene glycol (PEG) production, active pharmaceutical ingredient (API) production, and an automated aseptic filling plant. At every step, the PharmaEssentia manufacturing team meticulously prioritizes the quality and security of our products.


GMP production plant
Our main manufacturing facility at Central Taiwan Science Park in Taichung City includes a PEG production plant, a biologic drug substance production plant, and an aseptic filling plant. Two Drug Substance (DS) production lines provide capacity to support up to 40,000 patients.
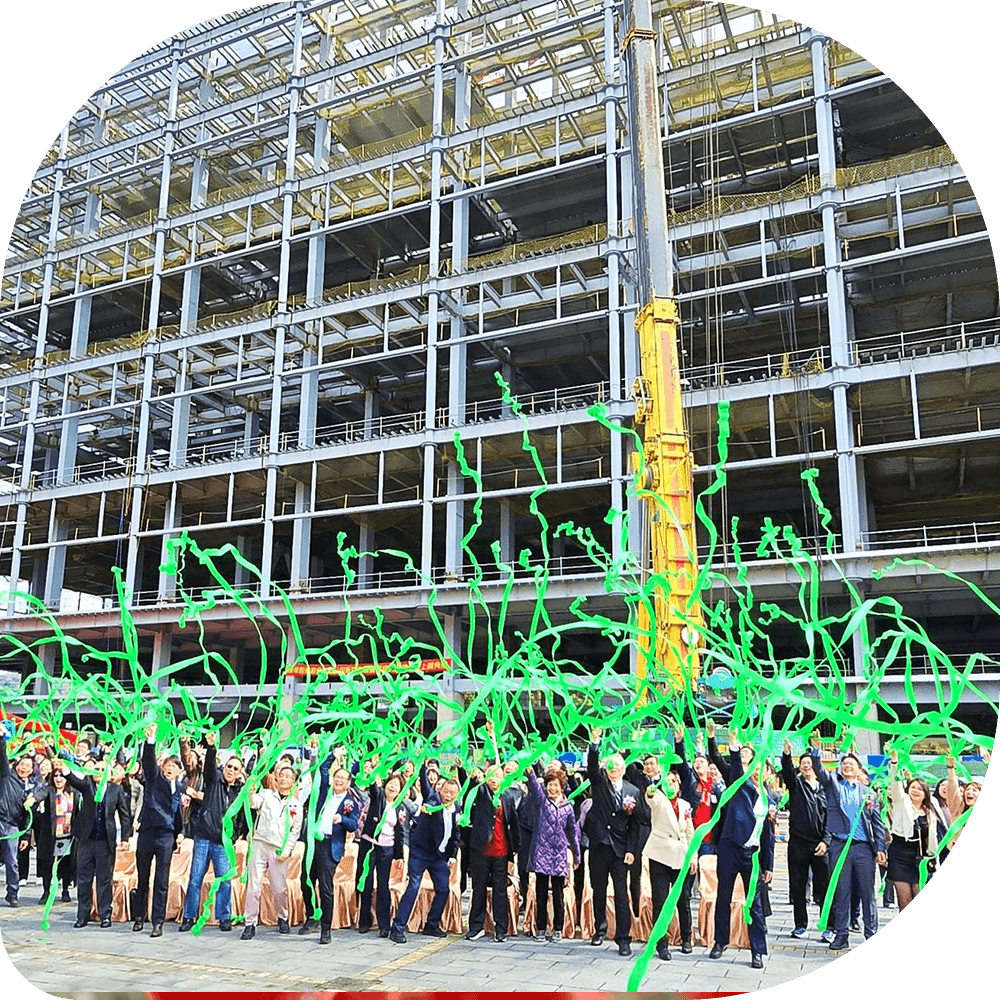
Expanding our capacity
Our new facilities, expected to be completed by 2026, will expand our production capacity to serve more than 100,000 patients worldwide. In addition, efforts are underway to expand components of our manufactuing efforts in the United States, including fill and finish.
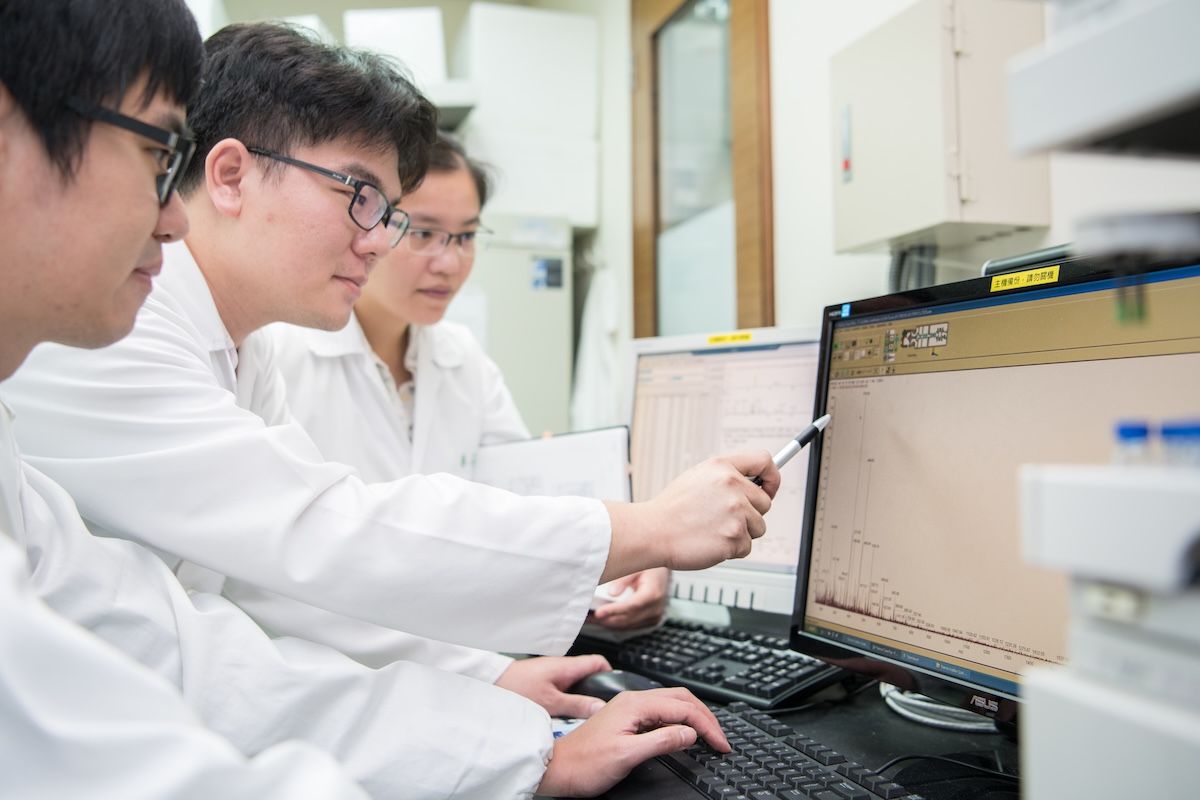
Quality management
PharmaEssentia is committed to ensuring the highest standards of product quality and patient safety. Our robust Quality Management System meets Good Manufacturing Practice (GMP) requirements. We are regularly inspected by the relevant authorities, including the US FDA, EMA, Taiwan FDA, and the PMDA.

Janice Hao
Senior Manager, Quality Assurance
Quality is at the heart of everything we do. From creating clear SOPs to ensuring compliance with global standards, our goal is to make sure every product we deliver is safe, effective, and consistent—because patients rely on us.